Detailed marine mammal necropsy guide
Introduction
Strandings of whales and dolphins have always intrigued people and must have occurred from the time cetaceans have been present in the ocean. Seals, having also a terrestrial behaviour, are well known and perhaps less intriguing. Mass stranding can be defined as an event where two or more animals run ashore alive at roughly the same place and time. Many theories have attempted to explain that phenomenon. In most cases, it cannot be attributed to a single cause, but is the result of a complex interaction of physical and biological factors such as ocean currents, tides and coastal configuration, the animals' migratory and social behavior, food availability, echolocation or orientation failure, and diseases which have debilitating effects.
All those considerations should be taken into account for the evaluation of the cause of the stranding. The emergence of new diseases such as Morbillivirus infections or new theories on stranding causes like anthropogenic activities (for instance pollution and sonar) justify to perform post-mortem evaluation of all stranded animals and multidisciplinary investigations to understand the processes and the health status of marine mammals populations.
Why necropsy
The aim of necropsies is to provide information about
- the cause of death
- the identification of lesions and their potential origin and with that the health status of and the mains threats to a population
- the collection of samples
It is always best to perform the necropsy as soon as possible after the standings but, in practice, this is not always possible. Indeed, in case of mass strandings or epizootics (Morbillivirus), there are too many animals and other solutions have to be found. To understand exactly what is happening, it is recommended to perform the necropsy of almost animals, if not all. The best solution is to freeze animals that can be not investigated directly to -20°C. This is only suitable for small cetaceans and pinnipeds.
The methodology presented below is organized in two parts:
- general considerations for marine mammals necropsy and
- particularities for small cetaceans, pinnipeds and large cetaceans.
General Procedure
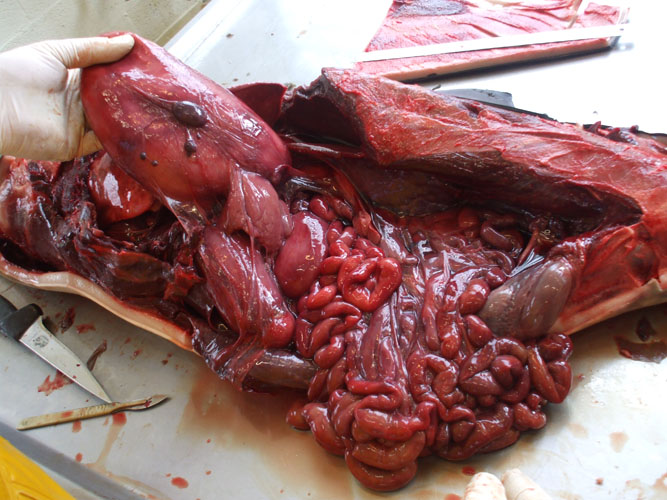
A necropsy reference number or code (one and only one) should be given for every animal, and the reference should be present on every samples collected. All organs must be carefully examined and sliced, ideally at regular intervals. Lesions must be described before and after incision (taking into account shape, colour, content, consistence, aspect,...), sampled (see below) and photographed with a ruler indicating the size and the necropsy reference of the animal. In addition to lesions, some tissues are systematically collected for histopathological, parasitological, bacteriological, virological, toxicological and live history investigations (see annex 3). This sampling can be performed without accurate order but tissues for bacteriology, virology and electron microscopy should be collected as soon as possible. In addition, to prevent contamination by micro-organisms from intestine, intestinal tract should be left intact until last.
External examination
Body condition is estimated using condition code (see table 3). Photographs should be taken of lateral view, more particularly of the head, body openings and external lesions.
Nutritional status is evaluated taking into account the blubber thickness and the muscle aspect; a bad nutritional state being characterized by a thin blubber layer and muscle atrophy (hallow aspect lateral to dorsal fin and protusion of lateral processes of lumbar vertebrae).
Skin is carefully examined for lesions and ectoparasites, particularly around body openings. Special attention should be paid to any scars (precise description with photographs, localisation, number, size,...) allowing animal's identification. A skin sample is taken for live history.
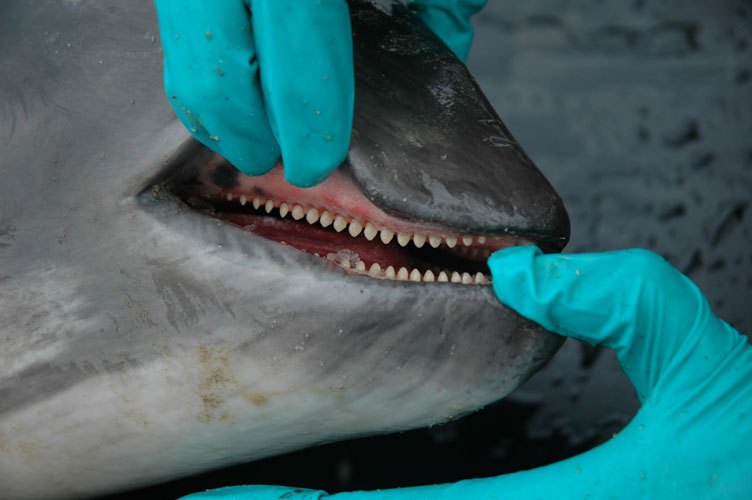
Body openings including mouth (tongue, palate, teeth or baleen plates), eyes, blow-hole(s), ear openings, genital slit and anus are examined, and any lesions, parasites or discharges are described and sampled. Pictures are taken when needed. In female, mammary glands are examined and sampled for histopathology. When present, milk is collected for toxicology and biochemistry. In neonates, the umbilicus is examined and collected for histology.
Abdominal cavity opening
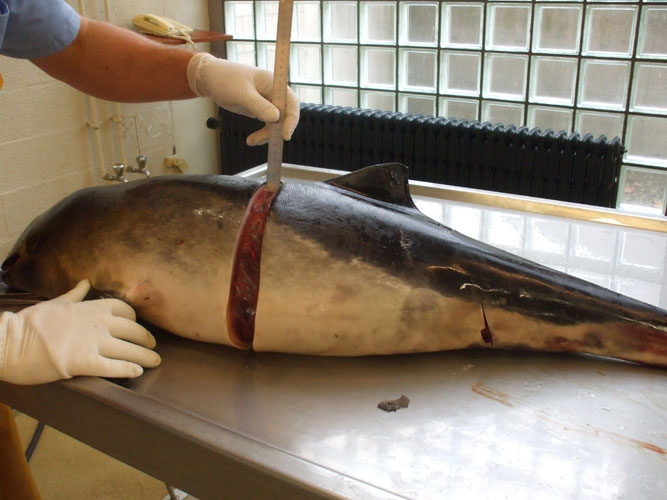
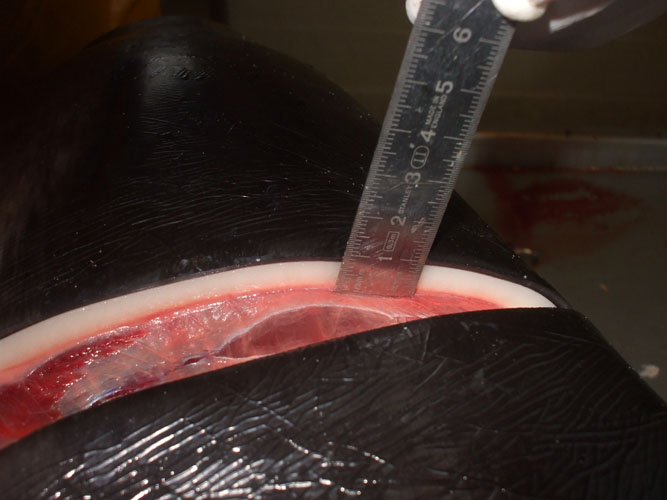
The blubber and its interfaces with skin and muscle are particularly investigated for the presence of parasites and parasite cysts. Some of these cysts have nearly the same colour as blubber fat and are not easily identified. Sampling for toxicology should be done at the caudal insertion of dorsal fin and should be performed when blubber thickness is measured.
Muscle is collected for toxicology (heavy metals and organochlorines) below the place of blubber sample (same place, same time).
The peritoneum is particularly investigated for the presence of parasites and parasite cysts.
Bacteriological and virological samples from abdominal organs should be collected as soon as the abdominal cavity is opened, with the exception of the intestine. Frequently, the gastrointestinal tract, dilated by putrefaction gases, protrudes out through the opening of the abdomen. In such case, incisions are made along the greater curvature of stomach compartments. The intestine is ligatured cranially close to the stomach and removed by dissection of mesentery. Care should be taken as the intestinal tract is easily torn.
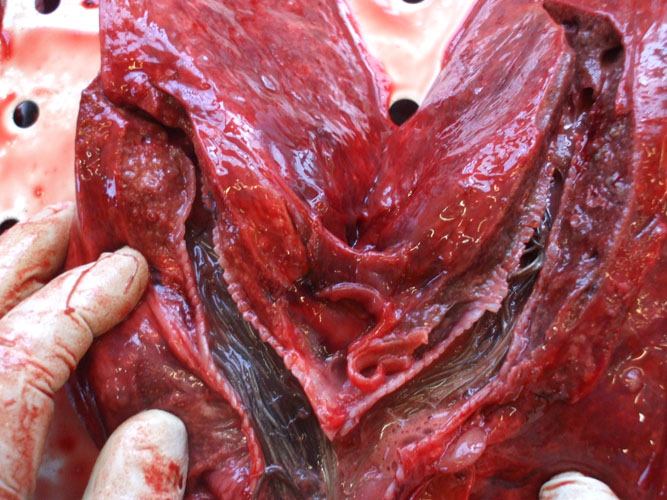
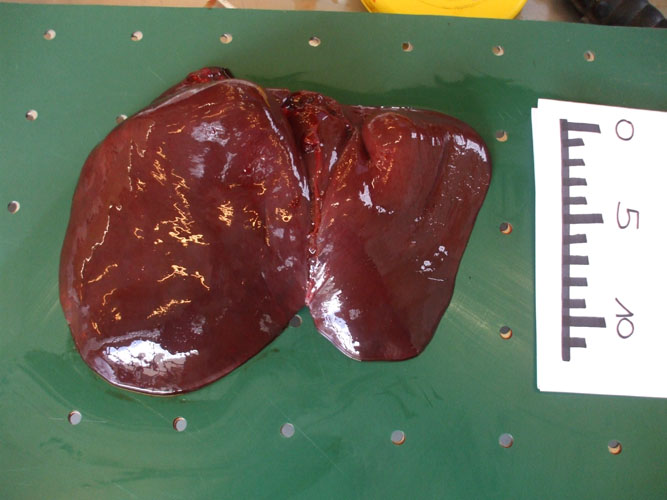
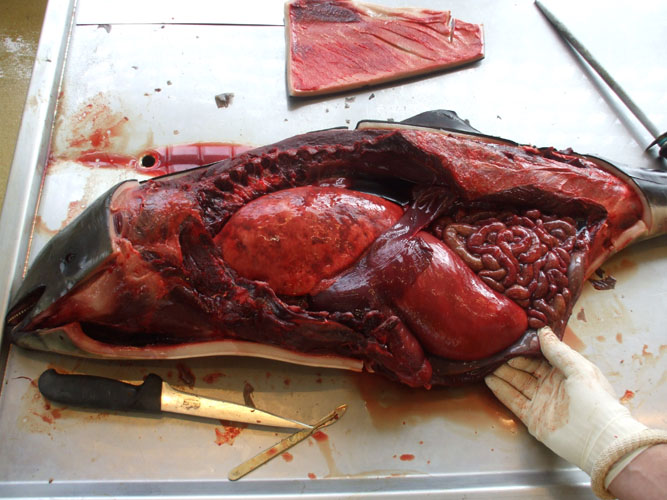
The liver is observed in situ on both side and sampled (toxicology -heavy metals and organochlorines, histopathology, virology, parasitology). The organ is then sliced for internal examination, at regular intervals, and the bile ducts are examined for the presence of parasites.
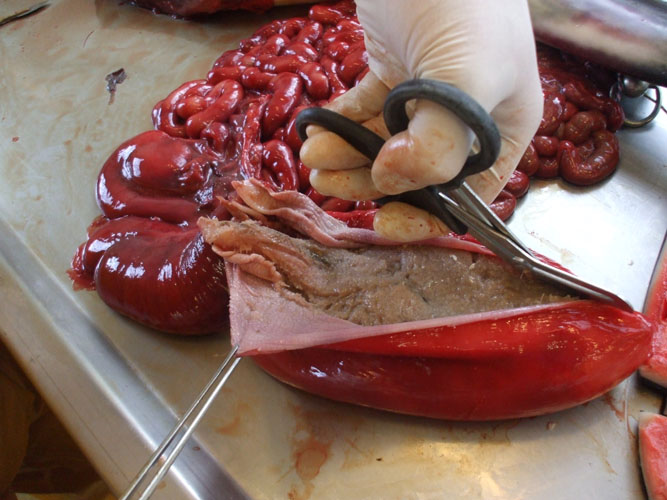
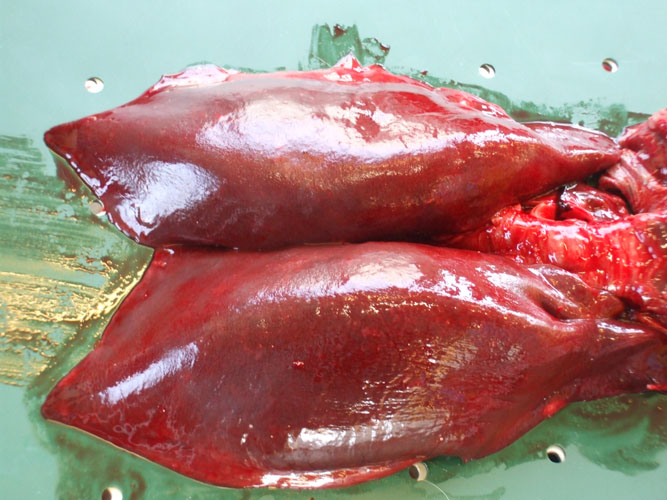
The kidneys are observed in situ, sliced at regular intervals and sampled (toxicology -heavy metals and organochlorines, histopathology, virology, parasitology) including complete reniculi with renal medulla and cortex. Renal tissue and blood vessels are particularly examined for the presence of parasites.
The urinary bladder is observed in situ and sampled for histopathology. Content is described (volume, colour,...) and should be collected at lower part of the bladder for parasitological examination (parasite eggs).
The gastric compartments are opened along greater curvature and content is directly collected (life history and parasitology). The stomach can be rinsed with water and the content sieved to collect the smaller parts (otolithe). Particular attention should be taken for the presence of parasites, lesions, otolithes, fishbones and cephalopod's beaks between gastric folds.
The testis are examined in situ, sliced and sampled for life history.
The ovaries are examined in situ, sliced and sampled for life history. Note any corpora lutea or albicantia or follicles on each ovary.
The uterus is opened and examined. In pregnant females, the foetus is examined and sampled according to the dissection protocol.
The adrenals (not easily observed) should be collected for histopathology. Particular attention should be draw for cysts. Cysts content should be collected (sterile syringe and needle) and kept frozen (-20°C) for biochemical analyses.
The pancreas is examined and pancreatic ducts are particularly examined for the presence of parasites. Tissue samples for histopathology need to be taken as soon as possible, as this organ is affected severely by decomposition.
The spleen and the mesenteric lymph nodes are examined and sampled for histopathology and virology.
The intestine should be examined last, outside of the abdominal cavity. Any lesion and all parasites should be carefully examined and collected. Intestinal content is described when present. Ligatured segments are collected for bacteriology and parasitology.
Thoracic cavity opening
If the skeleton is to be preserved, the opening of the thoracic cavity requires the detachment of the ribs from the vertebrae and from the sternum. As soon as possible, during this operation, samples for bacteriology, virology and electron microscopy should be collected. If skeleton is not to be preserved, ribs can be sawn through.
In the thoracic cavity, organs should be examined in situ, and after, the respiratory systems with the heart, major blood vessels and esophagus are removed from the thoracic cavity.
The lungs are examined, carefully described (size, colour, consistence, aspect,...) and sampled (histopathology, bacteriology and virology). Tissue is sliced at regular intervals and cut surface is examined for the presence of liquids (blood, serous,...) or foam oozing from parenchyma. Any parasite is collected.
The bronchioles, bronchi and trachea are examined and the lumen content, if present, is described. Any parasite is collected.
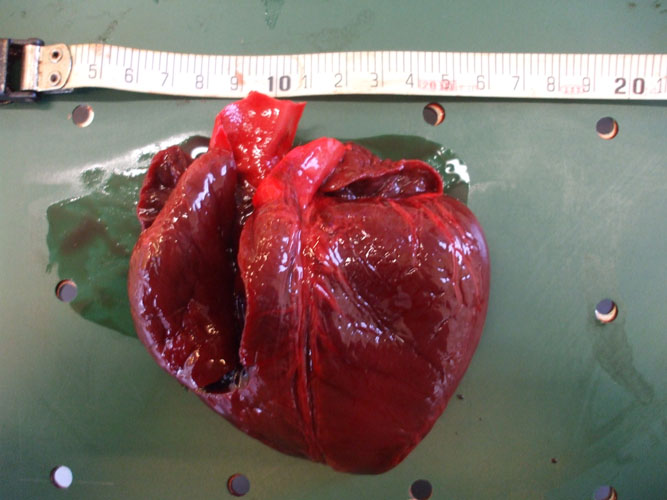
The heart is separated from the lungs by cutting the major vessels. Blood is collect from the heart lumen, from major vessels or from elsewhere for virology, for parasitology (blood parasites), for toxicology and for biochemistry. Left and right ventricles, and atria are opened and heart tissue is collected for histopathology. Any parasite is collected.
All major blood vessels are examined and any parasite is collected.
Neck and head
The lower jaw is dissected and cut at mandibular articulation (to be performed finally). The hyoid bones are cut close to the skull. By this way, all the buccal cavity can be examined as well as basis of the tongue with the tonsil area and larynx.
Dissection of this area allows examination of larynx, trachea and oesophagus. Oesophagus should be observed entirely for the presence of preys. When fishes are present, their orientation (head fish to stomach or to buccal cavity) must be specified. Dissection of tissue overlying trachea allows identification of thyroid gland and thymus (not easily observed). They are examined and sampled (histopathology).
Small Cetaceans
For cetaceans that are easily transportable, it would be easier to perform necropsy in dissection or necropsy room. Particularities and differences for small cetacean necropsy are presented hereafter.
External examination
Photographs should be taken of lateral view, more particularly for the presence of netmarks on the head, around rostrum and flippers. Pictures of the dorsal fin can be helpful for animal identification.
Body lengths (see annex ) are measured by placing tape next to the carcass (laying ventrally) in a straight line parallel to the longitudinal body axis. The total body length is taken from most cranial point of the rostrum to the notch in the tail fluke. The blubber thickness is measured after skin and blubber incision, vertically from the cranial insertion of the dorsal fluke to the ventral midline.
Externally, the penis is not visible, being wrapped in a subcutaneous sheath (preputial pouch), staying in the pouch by the contraction of a S-curvature. To examine the penis, it is necessary to pull it or to open the preputial pouch.
Abdominal and thoracic cavity opening
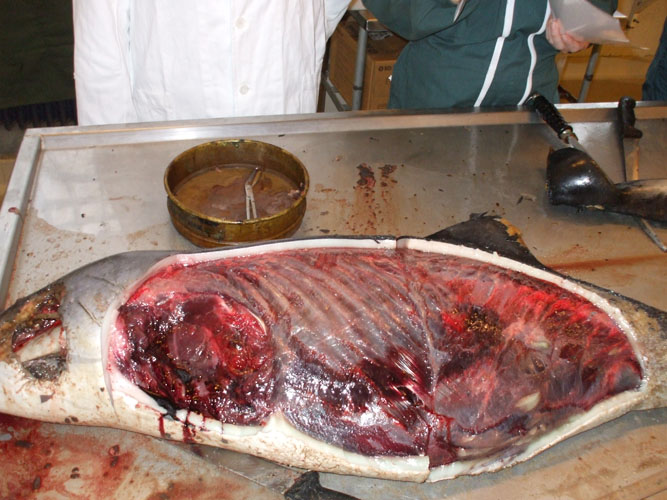
For practical reason, it is easier to open the animal by the left flank, the cetacean laying on its right and to open abdominal and thoracic cavities together. To open the cavities, one incision is made horizontally through the skin and the blubber, along the lateral processes of vertebrae from the head to the anus level. Two other incisions are made vertically from the first incision to the ventral midline, one between head and neck, one to the anus. The strip of skin and blubber is removed. To open the abdominal cavity, the landmark to find is the caudal extremity of the last rib. The extremity is pulled up and the abdominal wall can be opened by cutting muscles vertically along the last rib, without damage for intra-abdominal organs and caudally to the anus. The left thoracic breastplate is removed in one piece, dissecting the articulations between ribs and vertebrae and ribs and sternum. A rib can be collected for toxicology. Such opening allows to have a general overview of the topography and the organs in situ.
The examination of abdominal and thoracic organs and samplings of procedure are similar to what it is described in the general procedure.
Neck and head
The skin and subcutaneous tissues are removed ventrally from the intermandibular space up to the entry of the thorax. After examination and sampling of tissues (tongue, esophagus, trachea, thyroid, thymus), the head is separated from the rest of the body by dissection of the atlanto-occipital articulation. The lower jaw is separated and teeth (at least 4) are collected at the middle of the lower jaw and stored frozen for age determination. The acoustic fat (within the mandible) is examined in situ and the lower jaw is cut at the middle to detect any evidence of haemorrhage or acoustic fat bleeding. Acoustic fat is collected for histology.
Ear extraction and fixation protocol
Tympanic-periotic complex
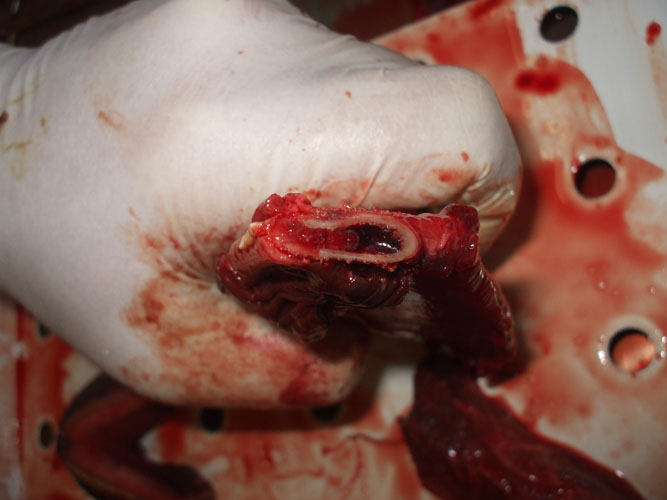
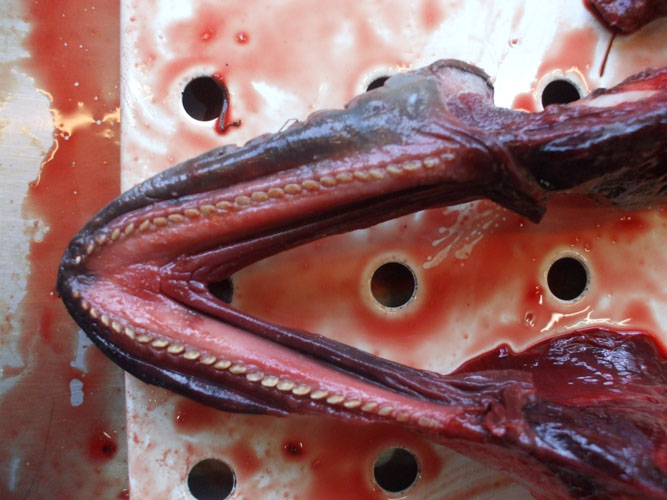
The tympanic and periotic bones house the middle and inner ear, respectively. These structures are partially fused forming the tympanic-periotic complex (Figure 1). The tympanic-periotic complex is surrounded by aerial sinuses called peribullar sinuses and suspended in the peribullar cavity through ligaments that hold it fixed and acoustically isolated it from the rest of the bones of the skull, except the sperm whales and some beaked whales who present the tympanic-periotic complex partially fused to the temporal bone.
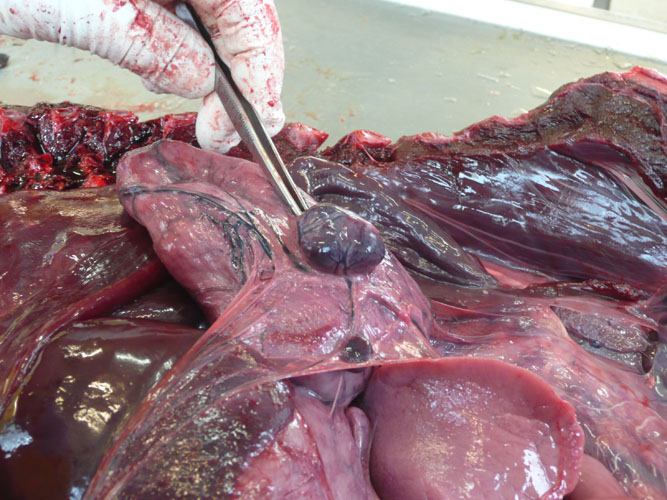
Figure 1.- Tympanic- periotic complex of a Stenella coeruleoalba stranded in Almeria, Spain. Sample provided by Promar
Extraction
1.- With small specimens, it is recommended to cut the head of the animal for an easier manipulation (Figure 2).
Figure 2.- The position of the tympanic-periotic complex and auditory external meatus is indicated. The dotted line marks the incision path to separate the head from the rest of the body. Alternatively, the digestive system can be extracted from the head to facilitate the access to the ears.
2.- Taking into account the localisation of the tympanic-periotic complex (Figures 3 and 4), the easiest way to access the ears is to carefully remove the lower jaw.
Figure 3.- Sagital cut of a bottlenose dolphin head where the location of the tympanic-periotic complex is indicated.
3.- Situating the head in a ventral position and removing the soft tissues and ligaments (Figure 4) allows to proceed to the tympanic-periotic complex extraction.
Figure 4.- Image taken during the necropsy of a Phocoena phocoena. This image reflects how the tympanic-periotic complex appears after removing the lower jaw (no effort has been made here to clean the area of extraction)
4.- Incise gently around the tympanic-periotic complex with a small knife (a scalpel can be used for the final stage of the extraction) to cut the ligaments that mantain the ears in the paraotic sinus (see Figure 5).
Figure 5.- Image taken during a Phocoena phocoena necropsy. The dotted line illustrates the location where the knife should be placed to extract the tympanic-periotic complex.
Fixation
5a.- At that stage, the ear could be fixed simply placing it in a fixative solution: glutaraldehyde 2,5% with phosphate buffer 0,1M 2 or a mixture of paraformaldehyde 0,5% with glutaraldehyde 1% with phosphate buffer 0,1M 3. If the mentioned fixative solutions are lacking, the tissues can be also fixed with formaldehyde 10%.
However, for a better result we recommend to follow the protocol described in point 5b.
5b.- If already experienced with the injection protocol, you may want to progressively and very slowly introduce the fixative solution (glutaraldehyde 2,5% with phosphate buffer 0,1M or a mixture of paraformaldehyde 0,5% with glutaraldehyde 1% with phosphate buffer 0,1M) through the oval window (after removing the stapes and making a small and superficial hole in both windows) and the round window (Figure 6) using a soft catheter from the same diameter as the windows size (Figure 7).
If the above fixative solutions are lacking, the ears can alternatively be injected with formaldehyde 10%.
The injection is a very delicate process and if you do not feel comfortable with it, please do not perform it. It is important to mention if the ear has been injected or not when sending it. In any case, before performing the injection, you are welcome to contact the Laboratori d'Aplicacions Bioacústiques.
Figure 6.- Localisation of the oval and round windows in the periotic bone.
Figure 7.- Tursiops truncatus periotic bone used to illustrate all the steps in the injection process: A) cut of the stapedial ligament, B) removal of the stapes, C and D) the performance of a little a superficial hole in the oval and round windows respectivelly, E and F) introduction of the fixative solution progressively and very slowly (very little pressure) through the oval window and the round window using a soft catheter from the same diameter as the windows size, until the solution gets out through the other one for some seconds.
6.- Place the ears in jars that contain the fixative liquids (see point 6).
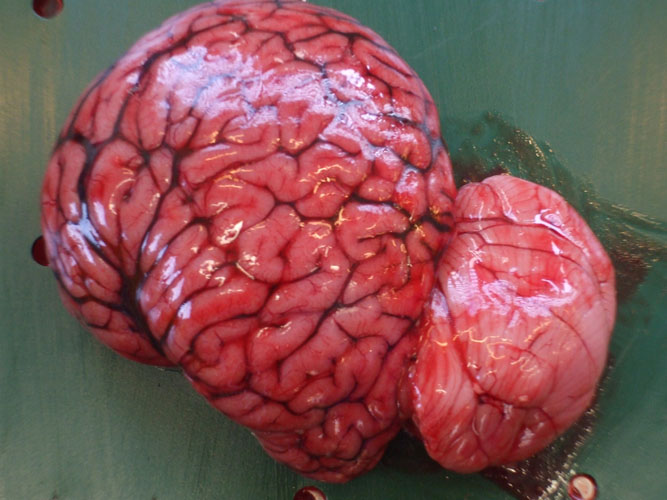
Brain or central nervous system tissue
If spinal cord sample is necessary (histology or microbiology), it can be collected after the head separation within the atlas vertebrae. To have access to the brain, the skull is sawed longitudinally. The blowhole and the nasal passage can be examined for the presence of parasite, liquid or foam. The pituitary gland (mid-ventral part of the brain, in the Sella turcica ) is sampled separately for histology. A complete cerebral hemisphere is collected for histology while the other hemisphere is sampled for virology, toxicology and bacteriology.
The peritympannic sinuses are dissected and examined for the presence of parasites and fluid.
To prepare 1 l of glutaraldehyde 2,5% is necessary to dilute 100 ml of concentrate solution (glutaraldehyde 25%) in 900ml of phosphate buffer 0,1M, prepared following this mixture: 3,12 g of NaH 2 PO 4 2H 2 O (PM =156 g) in 100 ml distilled water 28,64 g of Na 2 HPO 4 · 12 H 2 O (PM= 358 g) in 400 ml distilled water Add 500ml of distilled water to the mixture
Please, be aware that once these products are mixed, the fixative power is active for around a week; so it's better to keep them separately and do the mixture at the last moment.
Pinnipeds
Most of pinnipeds within European waters can be easily transportable and necropsy can be organized in good facilities such as dissection and necropsy rooms.
Particularities and differences for pinnipeds necropsy are presented hereafter.
External examination
Body lengths (see annex ) are measured by placing tape next to the carcass (laying ventrally) in a straight line parallel to the longitudinal body axis. The total body length is taken from most cranial point of the head to the extremity of the hind flipper. The blubber thickness is measured dorsally after skin and blubber incision.
As for cetacean, the penis is not visible and should be examined and sampled as described here in before.
Abdominal and thoracic cavity opening
Seals are laying dorsally before opening cavities. A first incision is made along the ventral midline from the intermandibular space to the anus and two incision from the xiphoid process of the sternum to the foreflipper (V-incision). Flap of skin and subcutaneous tissues are removed. Abdominal cavity is opened along the hypochondrium and after by two backward incision along dorsal muscles. Ribs are cut or sawed at mid-height and the thoracic breastplate is removed. A rib can be collected for toxicology. Such opening allows to have a general overview of the topography and the organs in situ.
The examination of abdominal and thoracic organs and samplings of procedure are similar to what it is described in the general procedure. The stomach is single.
Neck and head
After examination and sampling of tissues in the neck area (tongue, esophagus, trachea, thyroid, thymus), the head is separated from the rest of the body by dissection of the atlanto-occipital articulation. The lower jaw is separated and a canine tooth is collected and stored frozen for age determination.
Brain or central nervous system tissue
If spinal cord sample is necessary (histology or microbiology), it can be collected after the head separation within the atlas vertebrae. To have access to the brain, the skull is sawed longitudinally. Nostril and air passage can be examined for the presence of parasite, liquid or foam. The pituitary gland (mid-ventral part of the brain, in the Sella turcica ) is sampled separately for histology. A complete cerebral hemisphere is collected for histology while the other hemisphere is sampled for virology, toxicology and bacteriology.
Large Cetaceans
On spot organisation
Necropsy of large cetacean or many large cetaceans, in case of mass stranding such as frequently observed for sperm whales, present a major logistical and scientifical challenge if complete examination is to be performed. It needs an effective collaboration between public authorities, scientists, media and public. We considered as large cetaceans, animals that are too large to be necropsied out of the stranding site (money-, time- and manpower-consuming for transportation). Those are sperm whales, beaked whales and baleen whales.
Performing a gross pathological examination on the carcass of a large whale on the beach -to collect the aforementioned information and samples- requires the help of heavy equipment and considerable manpower. In touristic areas, like for instance most coastlines of the southern part of the North Sea, the beaches must be as clean as before the stranding and dissection, for both hygienic and economic reasons. It is -more often than not- not possible to let a whale carcass lie to rot where it is (and let tide, weather and scavengers do the work). Nor can it be towed out to sea in most cases, as whale carcasses may drift back to shore driven by currents, winds and tides, and floating large carcasses may represent a major danger for navigation. On site burying is hazardous for both the possible environmental contamination and the potential of whale carcasses "refloating" on the sand, e.g. unburrying themselves, during the process of decomposition. To date, there is no valid solution for on site carcass disposal and stranded animal(s) must be removed and disposed off elsewhere. Such work requires heavy equipment and manpower. For the necropsy, the best solution is collaborate with a team in charge of the carcass disposal, benefiting from their infrastructures. It should be considered potentially dangerous (dissemination of pathogens organisms,...) to open a whale carcass without this equipment useful for the elimination of whale's waste (muscle, blubber flats, visceral organs) from the beach. When material is not available or if collaboration is not directly possible, heavy material should be obtain from state, municipal public works department and from private sources.
The role of technical coordinator is essential for any operations on the beach. This person should coordinate all technical support, ensure the security of workers, press and by-standers alike, and communicate effectively with local and public authorities. The coordinator should ensure the collaboration with the team in charge of carcass disposal to obtain as soon as possible the heavy equipment without delay. Coordinators (scientific and technical) should consider all political and legal aspects. As there are many unexpected characters of such events, such coordination should be well prepared and not improvised on the spot. In area where stranding of large cetaceans or mass strandings frequently occured like the North Sea, preliminary meetings (national and international), out of event's excitation, with all intervening parties (coordinators, scientists, authorities,...) may facilitate field collaboration and coordination. This may be achieved best by a periodic meeting defining:
1) stranding interests -scientific, public exhibition,...-; 2) the local status of marine mammals; 3) the political and legal framework such national and international agreements; 4) the role of the research programs on marine mammals; 5) possible financial support for whale disposal; 6) technical availability. During such conferences, previous stranding experiences and results should be presented, and discussed on a round table to improve the stranding management and the protocols for necropsy and sampling.
With well-trained and well coordinated teams, necropsy is realised in good conditions and in a reasonable timeframe with a certain comfort. In addition, mass stranding of sperm whales require several necropsy teams; postmortem examination itself should only be performed by trained scientists. Such organisation requires collaboration between pathologists in charge of marine mammals necropsy, and help of volunteers like students in veterinary medicine or in biology. As previously described, such logistics should be prepared in advance and not improvised on the beach.
Stranding of one or many large cetaceans is very attractive for public and media, and major efforts should be done to give the right information to journalists. As soon as possible, co-ordinators and scientists must organized on the spot, a press conference with the results of beach investigations (first observations of animal(s), first necropsy results,...). A second press conference could be organized after short delay (after event excitation) with results available from laboratories involved in the network.
Necropsy of large cetaceans is very different to that of small marine mammals made in a well-equipped necropsy room. It is usually performed on the beach near the place of stranding. Basic necropsy equipment for large cetaceans should be ready and available beforehand. So, it is important to have an updated checklist of material to take on the spot (annex 1). It is useful to have phone lists to contact and mobilize: 1) personnel and volunteers; 2) public and local authorities; 3) Civil Protection and 4) and to obtain additional material when required (heavy equipment).
Field material should be assembled as kits, each being in is own transport box (table 1).
Plastic badges are usefull to identify people on the beach with name, institutional affilitation and function (coordinator, pathologist,...). Badges should also be provided to volunteers. In addition, for night work each person should have reflective safety tape.
Cetaceans might carry potentially pathogens which are potentially hazardous to humans (zoonoses). Appropriate clothes like waterproof dress such as those for fisherman, rubber cloves and eventually mask are particularly indicated.
Manipulation of large cetacean carcasses requires heavy and haulage equipment working on beach. Either, it is in collaboration with team in charge of carcass disposal or it should be provided from state, municipal public works department or from private source.
As soon as possible (before necropsy), an operation center with phone, fax and internet line close to the stranding site should be established (hotel, communal building,...) where coordination meetings, teams briefing, press conferences,... will be organized.
All intervention should be done with care for human safety. Large cetaceans stranding events usually cause major agitation and excitement of authorities, scientists, volunteers and public, the latter often extremely curious and sometimes willing to help. For safety reasons, the number of people with direct access to the carcass(es) should be restricted to working teams and local authorities (police, fire brigade, Civil Protection). The latter should limit the access with barriers at sufficient distance of animal (25 m mimimum) to allow the work of scientists and of heavy machines. On some animals in advanced decay, there is a real risk of explosion-like evisceration due to the elevated internal pressure following decomposition gases production. It is a supplementary reason to take public at significant distance of the animal. Identification badge helps police service to limit the access to allowed person.
It is possible to collect data and information by one person with one knife and one measuring tape. However, complete examination of a large cetacean, including necropsy requires manpower and heavy material (truck and bulldozer).
The second step of intervention is to avoid that carcass might drift away with the following high tide. The body can be dragged ashore at the maximum high tide and sand barriers can erected by bulldozer around the whale will delay the body immersion at the next tide.
Putrefaction and all associated postmortem tissues damages and alterations are very fast processes in large cetaceans and more obviously in sperm whales. Therefore, necropsy and tissues sampling should be performed as soon as possible without delay and require a quick, effective and save waste disposal.
Necropsy team & procedure
For one large cetacean and more obviously in case of a mass stranding, necropsy should be performed by a team of at least 3 persons, including a trained pathologist working with one assistant in charge of photographs and taking notes of necropsy observations, and one asssitant in charge of sampling (storage and labeling). Ideally, these assistants should have basic scientific knowledge. When more persons or qualified volunteers are present, it is usefull to have one person storing samples (dirty work) working with one in charge of labeling (clean work). Additional manpower is needed for the manipulation of organs. As soon as possible, animal should have reference number, useful for the sampling.
Before the necropsy, it is necessary to hold a briefing to define the general rules of action, safety and hygiene on the beach. All parties involved (coordinator, scientists, volunteers, authorities, carcasse disposal team,...) should participate at this briefing. Similarly, a debriefing after completion of the necropsy and carcass removal is recommended. It will allow to collect all informations (e.g. for press conference) and to thank all participants. A liaison person, usually the one of the coordinators, should maintain contact with all non-scientific parties involved after the event and inform them of scientific findings. Mass strandings
Each animal must have one (and only one) reference number and this reference number must be used for the labeling of samples.
Three levels of intervention can be defined following the number of animals involved, the accessibility to the stranding site, the availability of heavy equipment and manpower, the presence of necropsy teams and the body condition of the animals. Considering these various parameters, postmortem investigations can be more or less developped (see table 2). In case of a mass strandings of more than 5 animals occuring on not easily accessible site (e.g. sandbank), or when heavy equipment or necropsy teams are not present, intervention should be limited (level 1). In case of a mass stranding of 5 or less animals, occuring on easily accesible area with heavy equipment quickly available and one necropsy team by animal, intervention should not exluded any point of the present protocol (level 3).
In case of mass strandings of more than 5 cetaceans, necropsy should begin and be limited on animals in the best condition code.
When animals strand on sandbank at some distance of an accesible beach, they should be dragged ashore as soon as possible. This increases delay for interventions but should not exclude necropsy and tissues sampling. Carcasses disposal and necropsies are two different interventions performed by two different teams but they can operate simultaneously in collaboration. It is recommended to drag ashore first (if possible) the animal in good condition (see condition code), so the two teams can work directly.
Necropsy
All organs must be carefully examined and sliced, ideally at intervals of of 10-20 cm. Lesions must be described before and after incision (taking into account shape, colour, content, consistence, aspect,...), sampled (see below) and photographed with a ruler indicating the size. In addition to lesions, some tissues are systematically collected for histopathological, parasitological, bacteriological, virological, toxicological and live history investigations (see annex 3). This sampling can be performed without accurate order but tissues for bacteriology, virology and electron microscopy should be collected as soon as possible. In addition, to prevent contamination by micro-organisms from intestine, intestinal tract should be left intact until last. Precautions should be take to avoid sample contamination by sand (not easy on sandy beach), particularly for histopathology and toxicology.
External examination
Body condition is estimated using condition code (see table 3). Photographs should be taken of lateral view, of the dorsal and pectoral fin (ideally ventral view), plus body openings and lesions.
Body lengths are measured by placing tape next to the carcass in a straight line parallel to the longitudinal body axis. The total body lenght is taken from most cranial point of the head to the notch in the tail fluke. The blubber thickness is measured at the caudal insertion of the pectoral fin.
Nutritional status is evaluated taking into account the blubber thickness and the muscle aspect; a bad nutritional state being characterized by a thin blubber layer and muscle atrophy (hallow aspect lateral to dorsal fin and protusion of lateral processes of lumbar vertebrae).
Skin is carefully examined for lesions and ectoparasites, particularly around body openings. Special attention should be paid to any scars (precise description with photographs, localisation, number, size,...) allowing whale's identification, and for the presence of parasites between ventral throat grooves. A skin sample is taken for live history.
Body openings including mouth(tongue, palate, teeth or baleen plates), eyes, blow-hole(s), ear openings, genital slit and anus are examined, and any lesions, parasites or discharges are described and sampled. Pictures are taken when needed. In female, mammary glands are examined and sampled for histopathology. When present, milk is collected for toxicology and biochemistry. In large male cetaceans and particularly the sperm whale, the penis protudes soon after death due to the internal pressure building up in the body cavities; it can then be easily examined. Particular attention should be drawn to the external ear of both baleen whales and sperm whales : the outer ear opening and the meatus acusticus externus should be sampled for histopathology and microbiology, the ear plug in mysticetes is collected for life history.
Abdominal cavity opening
In case of animal in advanced decomposition (condition code 4, in sperm whales also condition code 3), it would be dangerous to open the abdominal cavity directly. The internal pressure should be diminished either by placing a pressure-relieve cut on whale back (stand out of the way of extruding visceral organs) or -in more advanced cases of bloating- by introduction of rigid pipes through abdominal wall. After relieving the abdominal pressure to avoid "explosion-like evisceration", such decayed animal should be investigated last in case of a mass stranding; internal examination -other than for life history samples- is of limited value.
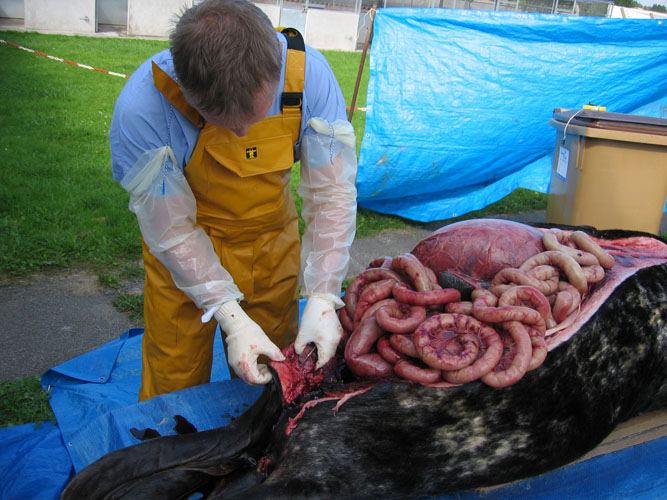
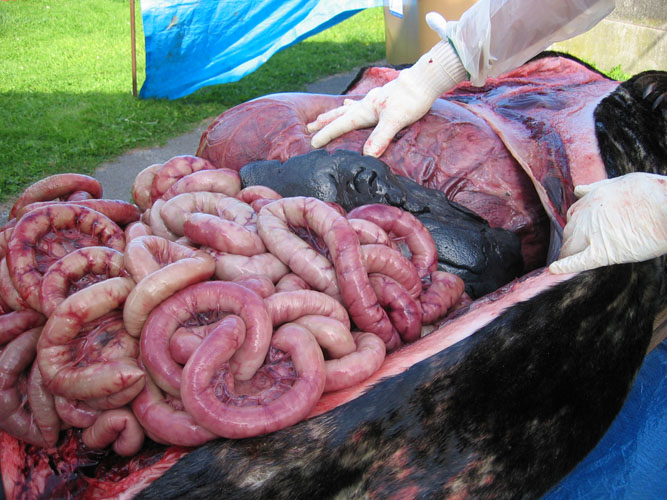
For abdominal cavity opening, it is necessary to cut a window in the belly. The skin and muscles are cut with relatively easily, but the rather fibrous blubber layer of sperm whales reaching 15 to 20 cm is very hard to incise. There certainely are several techniques to open the whale carcasses body cavities. As this is one (see figure) of the most time and strength consuming procedures during the entire necropsy, preference is given to the following technique and the use of bulldozers to pull long horizontal strips of skin and blubber; one incision is made through skin and blubber horizontally, at the mid height of the body, from the pectoral flipper to the anus level and two vertically, the first from the cranial part of the horizontal cut to the ventral midline and the second from the caudal part of the horizontal cut to the anus. The skin and blubber flaps are tied by ropes or chains at the horizontal incision and they are pulled by bulldozers (or men) for dissection of the abdominal blubber and exposure of abdominal muscles. A strip of tissue is removed and the subcutaneous tissue and blubber are examined for lesions and parasites. With the same technique, abdominal muscle layers and peritoneum are removed.
When it is necessary to climb onto the whale, it would be useful to cover whale's body with sand to avoid slipping.
While blood can be collected from the heart, blood samples from vessels of the skin or abdominal wall can be taken more easily and in most cases free of contaminations.
The blubber and its interfaces with skin and muscle are particularly investigated for the presence of parasites and parasite cysts. Some of these cysts have nearly the same colour as blubber fat and are not easily identified. Sampling for toxicology should be done at the caudal insertion of dorsal fin and should be performed when blubber thickness is measured.
Muscle is collected for toxicology (heavy metals and organochlorines) below the place of blubber sample (same place, same time).
The peritoneum is particularly investigated for the presence of parasites and parasite cysts.
Bacteriological and virological samples from abdominal organs should be collected as soon as the abdominal cavity is opened, with the exception of the intestine. Frequently, the gastrointestinal tract, dilated by putrefaction gases, protrudes out through the opening of the abdomen. In such case, incisions are made along the greater curvature of stomach compartments. The intestine is ligatured cranially close to the stomach and removed -while helpers are pulling the intestine out of the abdomen- by dissection of mesentery. Care should be taken as the intestinal tract is easily torn and it is relatively hard to pull it out of the abdomen.
The liver is observed in situ on both side as far as possible and sampled (toxicology -heavy metals and organochlorines, histopathology, virology, parasitology). The organ is then sliced for internal examination, ideally at interval of 10 cm, and particularly the bile ducts are examined for the presence of parasites.
The kidneys are observed in situ, sliced at interval of 10 cm and sampled (toxicology -heavy metals and organochlorines, histopathology, virology, parasitology) including complete reniculi with renal medulla and cortex. Renal tissue and larger blood vessels are particularly examined for the presence of parasites.
The urinary bladder is observed in situ and sampled for histopathology. Content is described (volume, colour,...) and should be collected at lower part of the bladder for parasitological examination (parasite eggs).
The gastric compartments are opened along greater curvature and content is directly collected. Particular attention should be taken for the presence of parasites, lesions and cephalopod's beaks between gastric folds. If the stomach seems empty, incision should be made at the sloping area to collect the liquid content (life history and parasitology).
The testis are examined in situ, sliced and sampled for life history
The ovaries are examined in situ, sliced and sampled for life history. Note any corpora lutea or albicantia or follicles on each ovary.
The uterus is opened and examined. In pregnant females, the foetus is examined and sampled according to the dissection protocol.
The adrenals (not easily observed) should be collected for histopathology. Particular attention should be draw for cysts. Cysts content should be collected (sterile syringe and needle) and kept frozen (-20°C) for biochemical analyses.
The pancreas is examined and pancreatic ducts are particularly examined for the presence of parasites. Tissue samples for histopathology need to be taken as soon as possible, as this organ is affected severely by decomposition.
The spleen and the mesenteric lymph nodes are examined and sampled for histopathology and virology.
The intestine should be examined last, outside of the abdominal cavity. The opening of large intestinal segments on the beaches should be avoided (potential micro-organism and waste dissemination). Such investigation can be performed in the mechanical shovel of the bulldozer before being discarded in the container for carcasses disposal. Any lesion and all parasites should be carefully examined and collected. Intestinal content is described when present. Ligatured segments are collected for bacteriology and parasitology.
Thoracic cavity opening
If the skeleton is to be preserved, the opening of the thoracic cavity requires the detachment of the ribs from the vertebrae and from the sternum. At first, it is necessary to strip the ribs from muscles and connective tissue; then, each rib has to be detached one by one (this procedure is very time-consuming). As soon as possible, during this operation, samples for bacteriology, virology and electron microscopy should be collected. If skeleton is not to be preserved, ribs can be sawn through. (If a complete opening of the thoracic cavity is impossible, for instance due to lack of time or of manpower, there are two alternative ways to open the thoracic cavity : after removal of the abdominal organs, the thoracic cavity is approached from the abdomen by cutting through the diaphragm, and cranially -after severing the head from the body, through the cranial thoracic opening. Alternatively, for a fast access to one lung, the thoracic cavity is opened by cutting a window between two ribs. This may allow visual examination and palpation of a very small part of one lung.)
In the thoracic cavity, organs are not easily examined in situ. Depending on circumstance, it may be usefull to remove the respiratory systems with the heart and major blood vessels from the thoracic cavity. Otherwise, examination and sampling are performed in the cavity.
The lungs are examined, carefully described (size, colour, consistence, aspect,...) and sampled (histopathology, bacteriology and virology). Tissue is sliced at regular intervals and cut surface is examined for the presence of liquids (blood, serous,...) or foam oozing from parenchyma. Any parasite is collected.
The bronchioles, bronchi and trachea are examined and the lumen content, if present, is described. Any parasite is collected.
The heart is separated from the lungs by cutting the major vessels. Blood is collect from the heart lumen, from major vessels or from elsewhere for virology, for parasitology (blood parasites), for toxicology and for biochemistry. Left and right ventricles, and atria are opened and heart tissue is collected for histopathology. Any parasite is collected.
All major blood vessels are examined and any parasite is collected.
Neck and head
When possible, lower jaw is dissected and cut at mandibular articulation (to be performed finally). The hyoid bones are cut close to the skull. By this way, all the buccal cavity can be examined as well as basis of the tongue with the tonsil area and larynx.
Dissection of this area allows examination of larynx, trachea and oesophagus.
Oesophagus should be observed entirely for the presence of preys. When fishes are present, their orientation (head fish to stomach or to buccal cavity) must be specified.
Dissection of tissue overlying trachea allows identification of thyroid gland and thymus (not easily observed). They are examined and sampled (histopathology).
4.5. Brain or central nervous system tissue
For the transportation of animal to process plant (carcasses disposal), the body is usually cut in 2 or more pieces. It is possible to collect brain tissue (histopathology, virology) through the occiputal formen once the head is detached (classical technique on sperm whale). It is possible to obtain the spinal cord when body is cut elsewhere.
Animal weight and blubber thickness
The total body weight should be calculated after weighing the carcass and necropsy wastes at the process plant at the time of carcasses disposal. For sperm whales, there is a correction factor (1.14) to compensate loss of body fluid during dissection and transport. A predictive formula of normal weight (W) can be used from measured length (L) -W=0.00218L 2.74 -. Such calculation allows comparison of estimated weight of the stranded sperm whales and weight reported on sperm whales caught during whaling operation. Similarly, blubber thickness can be compared with reference values. All those information are complementary to the evaluation of nutritional status (see above).
References
DIERAUF L. A., Hanbook of marine mammals medicine : health, disease, and rehabilitation. CRC Press, second edition Boca Raton, 2001.
GERACI J. R. & LOUNSBURY V. J. Marine mammals ashore a field guide for strandings. Texas A&M sea grant publication, Second edition, Galveston, 2005, p. 133.
JACQUES T. & LAMBERTSEN R. eds Sperm whale deaths in the North Sea : Science and Management, Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie, vol. 67 - supplément, 1997.
Jauniaux T., Garcia Hartmann M., Haelters J., Tavernier J. & Coignoul F. Echouage de mammifères marins : guide d'intervention et procédures d'autopsie. Annales de Médecine Vétérinaire, 2002, 146, 261-276.
Jauniaux T., Bouquegneau J.-M. & Coignoul F. eds, Marine Mammals, Seabirds and Pollution of Marine Systems, Presse de la Faculté de Médecine Vétérinaire, Université de Liège, Liège, 1997.
KUIKEN T. & GARCíA HARTMANN M. Proceedings of the first ECS workshop on Cetacean pathology: dissection techniques and tissue sampling. ECS Newsletter #17 Special issue, 1991.
LAW R. J. (compiler) Collaborative UK Marine Mammal Project : summary data produced 1988-1992, Fisheries Research Technical Report n° 97, Ministry of Agriculture, Fisheries and Food Directorate of Fisheries Research, Lowestoft, 1994.
LOCKYER C., Body composition of the sperm whales Physeter macrocephalus, with special reference to the possible functions of fat depots. Rit Fiskideilar Journal of the Marine Research Institute Reykjavik, 12: 1-24.
REYNOLD, J. E. & ODELL D. K., Proceedings of the second marine mammal stranding workshop, NOAA technical report NMFS 98, U.S. Department of commerce, 1991.